You didn’t get your flu shot. What should you do?
I strongly urge you NOT to get the flu shot. Three reasons:
1. It is too late for this season for the flu shot. The flu season is almost over and so your chances of getting it are small. Look at this graph from the CDC website . Along the Y-axis are the number of patients who go into the hospital for influenza (the flu) per 100,000 people. The X-axis is the CDC’s version of the calendar. They start on January first with week 1 and it goes from there. So what we call Thanksgiving week, a time of food, fun, and football for the rest of us, those party animals at the CDC call ‘Week 48.’
Each different colored curve is the flu season for a given year. So you see a pattern over the years. Beginning December 1, the number of cases begins. From January 1st to about March 15th (week 10) you see the most rapid weekly increases. This is what the rest of us call ‘flu season.’ From March 15th to May 7th (week 17) the cases are tapering off as spring begins, things start to get warmer, and we spend more time outside (this virus is a vampire; show it to the sun and it explodes!).
Notice the worse flu season recently was the 2017-2018, about 50% worse than this year, when over 100,000 people were hospitalized with 61,099 flu related deaths! I bet you didn’t hear about that then, did you?
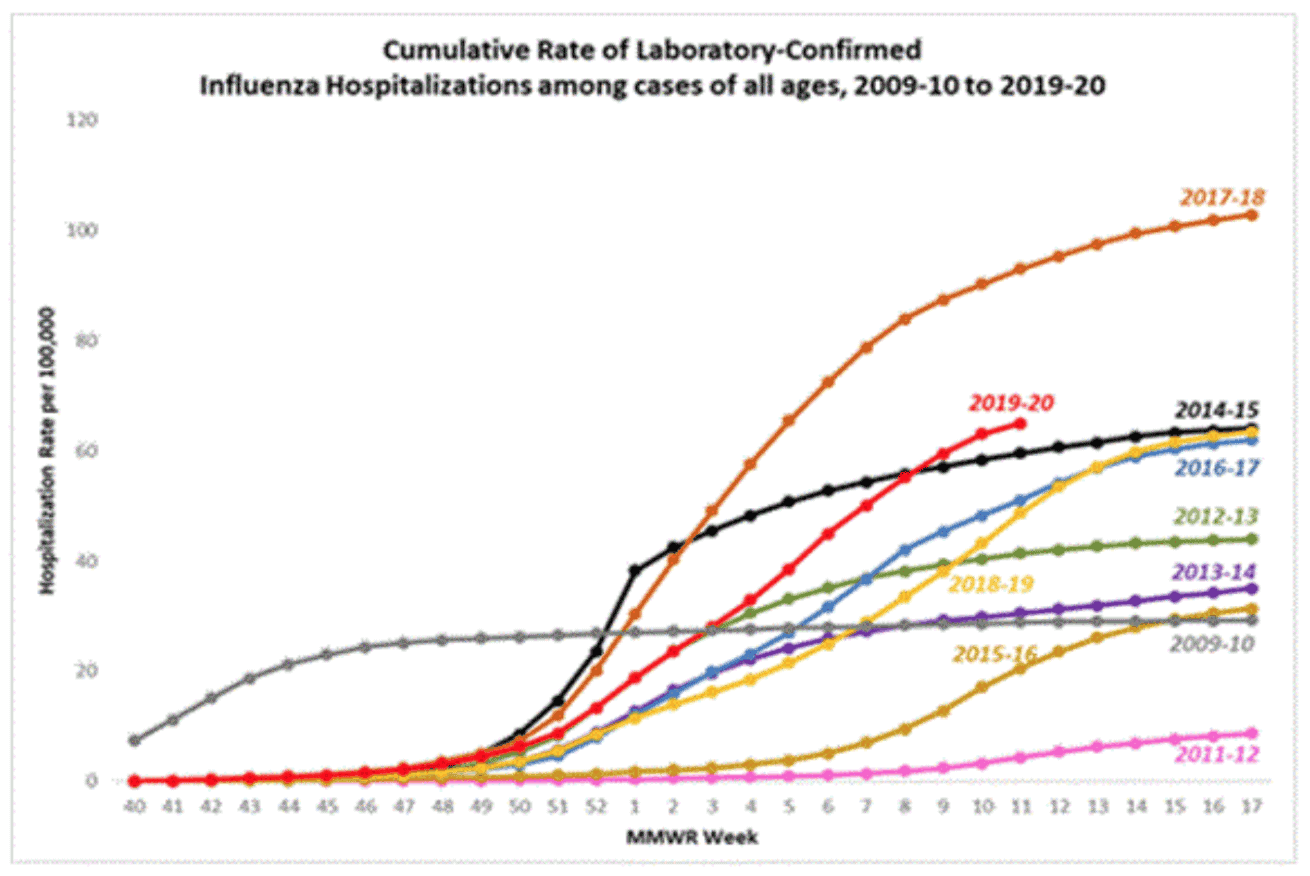
Back to the present. The flu season for 2019-20 is that incomplete red line (news flash; the CDC cannot predict the future and so the line stops with this week) is literally over with less than 10% of the cases left in the season.
2. Exposure to COVID-19 in medical facilities. You have to go to the pharmacy, local clinic, doctor’s office, or hospital to get a flu shot. These are exactly the locations where all the cOVID-19 patients are going when symptoms begin. A US-educated physician colleague of mine living in Shanghai says the medical community rumor there is that it takes 15 seconds in a room with a COVID-19 patient to become infected.
3. Free up non-essential medical resources. As a community we need to allow the medical community to focus on this pandemic for the next few weeks or months while it is still growing so fast. So all elective procedures, routine check-ups, wellness evaluations, etc. should be postponed until this crisis resolves.
This data can be found here: https://www.cdc.gov/flu/weekly/#S2